Novel regenerative platforms to grow surrogate organs using stem/progenitor cells
Abstract
Irreversible organ failure remains a worldwide concern as demand for transplantable organs far outpaces the available supply. Numerous tissue types have been successfully engineered in vitro, yet clinical translation has been achieved only for thin tissues or those with a low metabolic demand. Vascularization for large solid tissues or organs with higher metabolic requirements still remains a challenge. In an effort to overcome this limitation, we have pioneered an in vivo vascularized tissue-engineering model, in which target cells/tissues are implanted into a lymph node (LN). The LN —a secondary lymphoid organ, acting as filter for foreign particles and cancer cells— rapidly responds to accommodate increases in cellularity and has immediate vascular access, prerequisites for successful development of functional organs.
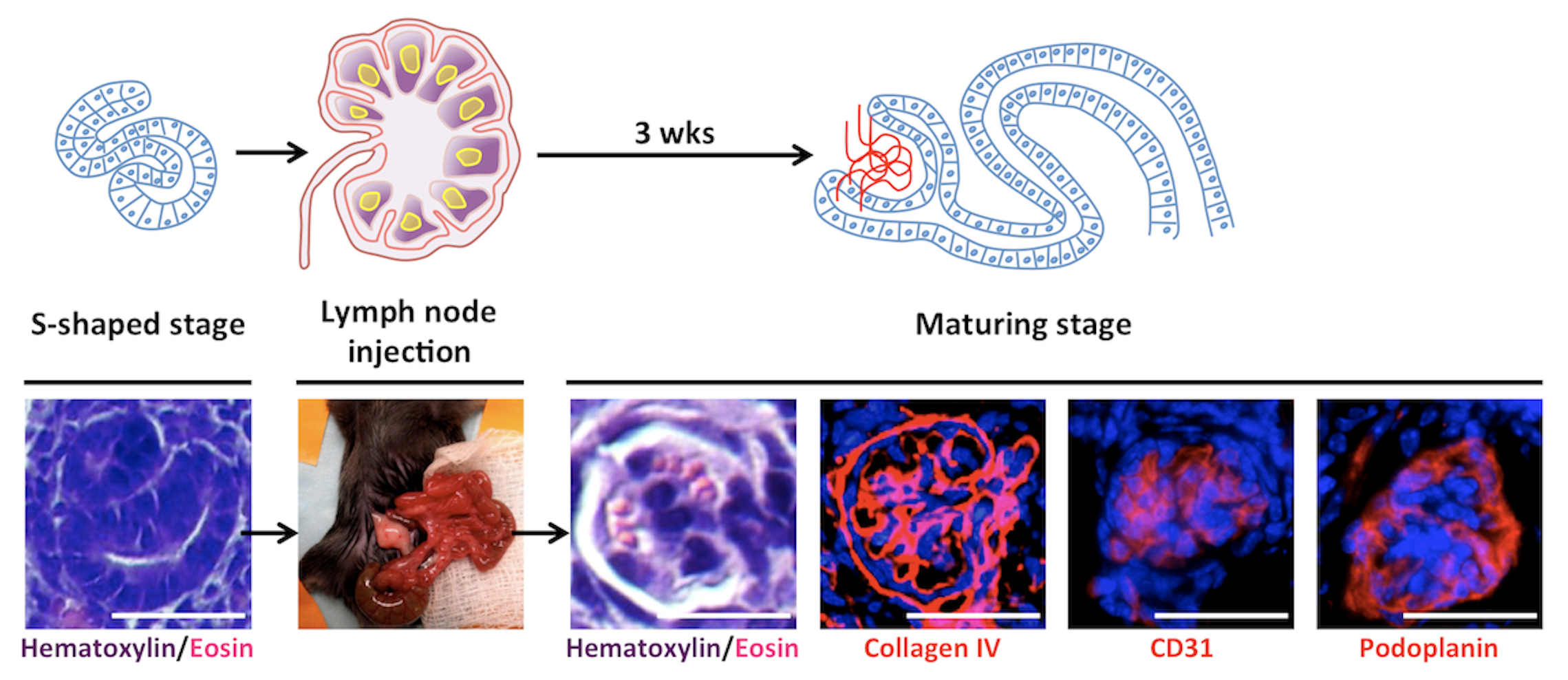
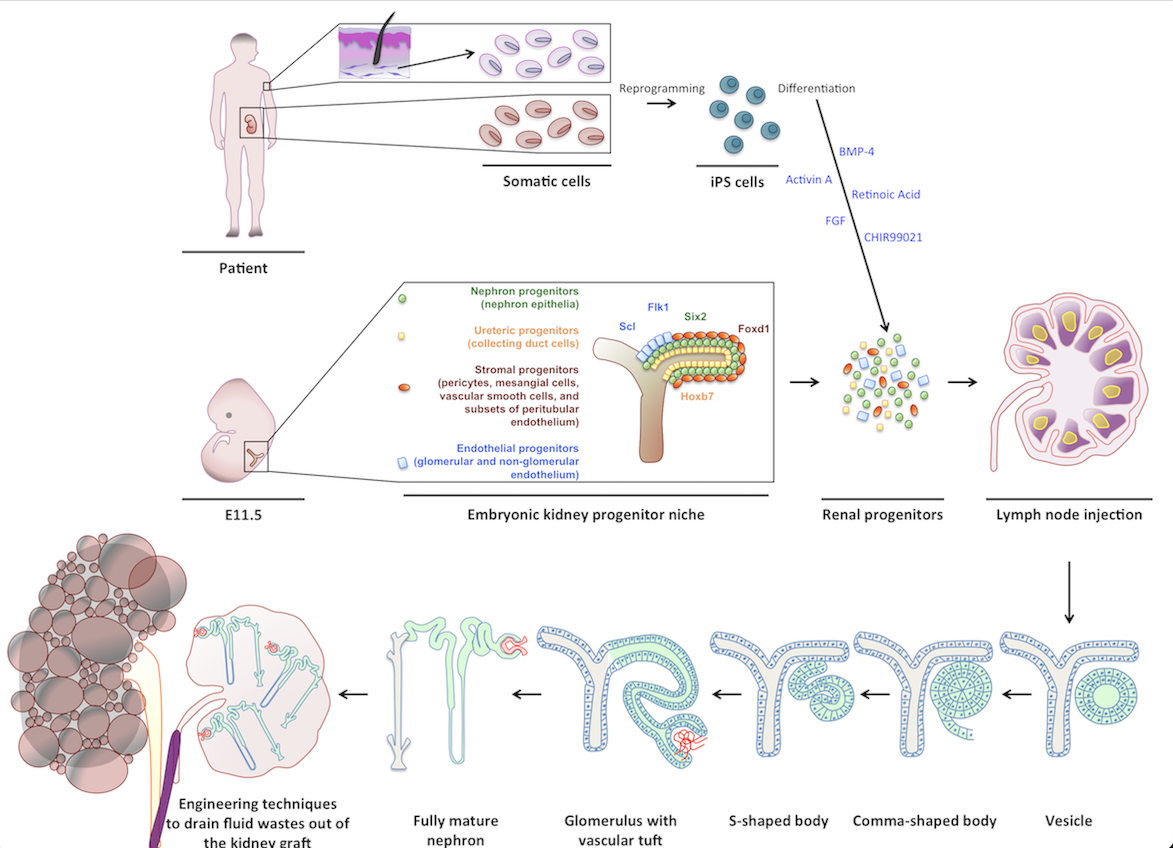
Initially, I investigated the LN’s ability to support the maturation of several embryonic tissues. Later, my interest focused on kidney tissue engineering using the LN bioreactor. Of all organs, kidneys are the most in demand for transplants. Current experimental approaches including the re-population of decellularized kidney scaffolds have failed to generate mature and perfused renal tissues that produce urine and/or have endocrine functions. My research showed that when transplanted into a LN, both mouse and human kidney rudiments mature in renal structures with excretory, homeostatic, and endocrine functions. My study also indicated the potential of using the LN as a platform to test the fate of newly emerging cell sources in kidney tissue engineering. Indeed, both mouse nephron progenitors and human induced pluripotent stem cells that were directed toward a renal fate in vitro, engrafted and matured in the LN. I also investigated the cellular/molecular mechanisms driving kidney organogenesis in the LN and the omentum, another lymphoid site. My data indicated that the LTbR signaling in the lymphoid stromal microenvironment is critical for successful angiogenesis of the transplanted renal tissues in these locations.
Similar to LNs, abdominal fat-associated lymphoid clusters (FALCs) can support organ regeneration. Particularly, our work has demonstrated the possibility to form, in both LNs and FALCs, functional ectopic livers that are able to rescue tyrosinemic animals from liver failure.
Collectively, these studies have resulted in issuance of two patents: “Lymph node as a site for transplantation, organogenesis and function for multiple tissues and organs” (E. Lagasse, inventor; Publication number: US20160058794; Publication date: March 3, 2016), and “Fat-associated lymphoid clusters as sites for transplantation, organogenesis and function for multiple tissues” (E. Lagasse, inventor; Publication number: US20190374583; Publication Date: December 12, 2019). This work has also led to the establishment of LyGenesis Inc, a University of Pittsburgh spin-out company (E. Lagasse, CSO; M. Hufford, CEO; P. A. Fontes, CMO) for which I have volunteered. LyGenesis’ lead preclinical program is focused on liver regeneration using a patient’s LN to restore hepatic functions. Liver cell injection into LNs worked in mice, where the surrogate mini-livers made up for the missing function of a diseased liver. Our preclinical studies in pigs were encouraging too, and at the time I was volunteering at LyGenesis (July 2019- July 2020), the company was completing an Investigational New Drug (IND) preclinical study in dogs, which was required to initiate the human clinical trial. At LyGenesis, I helped isolate Good Manufacturing Practice (GMP)-grade canine hepatocytes. LyGenesis received FDA clearance to begin Phase 2a trial of allogenic hepatocyte transplantation into periduodenal LNs for patients with end stage liver disease in December 2020 and is currently recruiting patients (ClinicalTrials.gov Identifier: NCT04496479).
LyGenesis’s drug development pipeline also includes cell therapies that can produce an ectopic thymus. In this regard, my work established a relationship between the amount of thymus tissue transplanted in LNs of athymic mice and the amount of newly-generated T cells in these mice. My results also suggested that males are less effective than females in producing new T cells after thymus transplantation. Importantly, low-dose thymus in aged wild type females rejuvenated T cell function, by ameliorating CD4+ naïve T cell percentages at the expense of CD4+ effector/memory T cells. This study has resulted in issuance of the patent: “Autologous thymic tissue transplantation” (E. Lagasse, inventor; Publication number: US20220152114A1; Publication date: May 19, 2022).
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contatto
Aree terapeutiche:
Prodotto:
Organi artificiali
Collaborazioni:
University of Pittsburgh, Department of Pathology. Pittsburgh, Pennsylvania, USA.
McGowan Institute for Regenerative Medicine. Pittsburgh, Pennsylvania, USA.
Mayo Clinic. Rochester, Minnesota, USA
Saint Barnabas Medical Center. Livingston, New Jersey, USA
Scarica il pdf del progetto