Tissue Engineered Vascular Graft (TEVG)
Brief description
Main topic: TEVG, development of engineered vascular graft for coronary bypass.
Solutions that are clinically available to replace or treat a stenotic blood vessel include the auto-transplant ,for example utilizing a section of the saphenous vein, or the adoption of synthetic materials such as Dacron or Teflon. The first class of intervention is limited by the availability to harvest sufficient viable autologous tissue. The second category utilizes synthetic materials that induce re-stenosis of the vessel up to the 50% of the treated cases. These issues could be potentially addressed by the tissue engineering approach.
The tissue engineering paradigm proposes the use of biodegradable scaffolds able to induce in-situ regeneration and lead to the formation of autologous, functional, non-thrombogenic tissue. In this research line our group has identified two main targets:
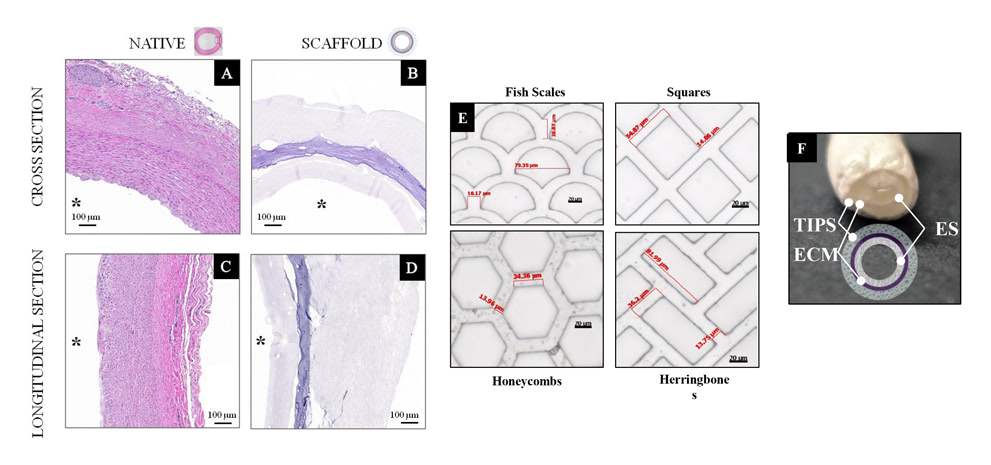
1) to design grafts able to reproduce structure and mechanics of the native tissue;
2) to reduce the tunica intima hyperplasia by the adoption of ad-hoc scaffold surface morphology ad structure.
Impact:
The main target of this research line is to introduce innovative strategies and technologies for coronary bypass and for the treatment of critical limb ischemia. Given the limitations of current artificial vascular grafts and surgical procedures, the introduction and validation of a technology based on biodegradable graft capable to promote in-situ tissue growth has a profound innovative value as well as a potential commercial value. Applications involved with the development of this technology extend far beyond the coronary bypass. Other examples include engineered urethra or the endothelialization of cannula utilized in FDA class II and III medical devices.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic area:
Products:
Medical devices & tissue engineering
Collaborations:
- Ospedale Cervello – Villa Sofia, Palermo, Italia
- University of Pittsburgh Medical Center, Pittsburgh, USA
- University of Pittsburgh, USA
- ATeN Center, Università degli Studi di Plaermo, italia
- LivaNova PLC, Londra, Regno Unito
Scarica il pdf del progetto
