
Development of a novel Alfa-Gal Free Xenograft heart valve
Brief description
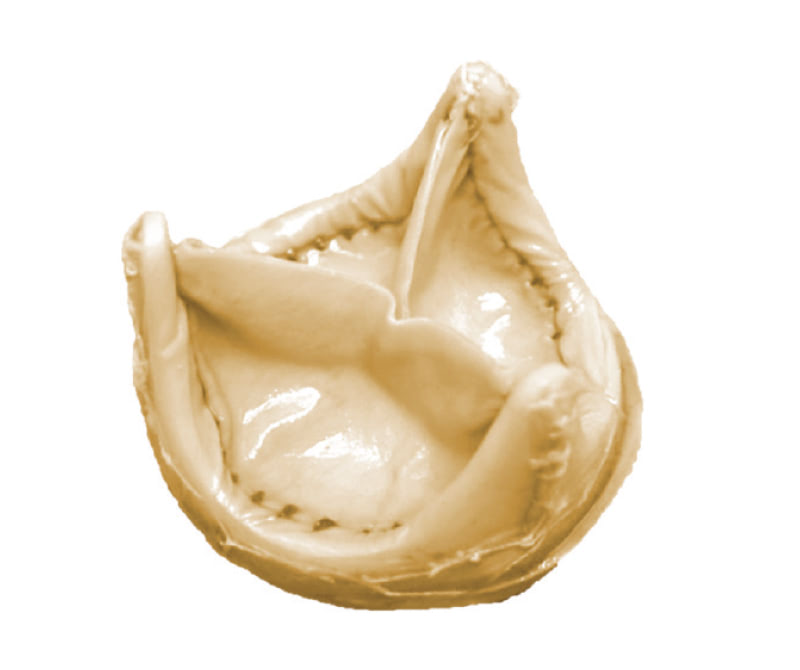
Bioprosthetic heart valves fail because they build up calcium deposits which weaken the valve, leading to tears, or obstruct blood flow because they impair the opening of the valve. Scientists and commercial valve companies have long sought to produce bioprosthetic heart valves which do not calcify, because these could be used in younger patients without the need for blood thinners. However, the calcification blocking treatments which have been developed so far have not been successful in younger adults. Our partners at UCL and UAB have identified an immune driven inflammation which accelerates calcification of biological heart valve materials. This inflammation is unique to humans because a portion of our immune system reacts with a substance called Gal, not made by humans but common in animals (including pigs and cows) and present on the bioprosthetic tissue.
To block this immune inflammation, our partners have genetically altered pigs, so they no longer make Gal.
Now, we are using the pericardial tissue from this new class of animals to develop a bioprosthetic heart valve which resists calcification, broadening the patient population suitable for bioprosthetic valves and improving the quality of life of recipients who receive this improved therapy.
Impact:
Approximately 300,000 valve replacements are performed annually worldwide. Two types of replacement valves are available, mechanical heart valves which require lifetime anticoagulation and bioprosthetic heart valves (BHVs) made from biological tissues, typically human or porcine heart valve leaflets, or animal pericardium. BHVs are preferred in older patients (> 65 years), where they are more durable. Younger patients generally receive MHVs due to rapid age-dependent BHV degeneration. In patents under 35 years of age, up to 100% structural valve deterioration occurs within 5 years. More durable BHVs would advance the standard of care by eliminating the need for anticoagulation in younger recipients and extending access to this therapy to more patients.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic areas:
Products:
Medical devices & tissue engineering
Collaborations:
University College London (UCL), Londra, Regno Unito
University of Alabama, Birmingham, Stati Uniti d’America
Scarica il pdf del progetto
