Development of an ex vivo stimuli-responsive osteoarthritis model for drug testing
Brief description
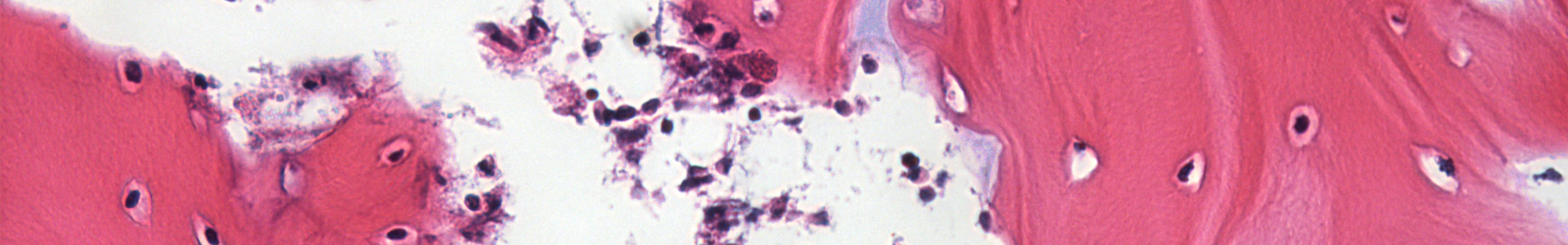
Osteoarthritis (OA) is a multifactorial disease involving all joint tissues causing degeneration of articular cartilage, subchondral bone sclerosis and synovial inflammation.
The tissues damage seriously compromise the overall functionality of the articulation, culminating in joint pain and disability. Osteoarthritis is characterized by a complex etiology which both genetic and acquired factors seem be involved in. In particular, it is well note as the OA onset is connected to pro-inflammatory stimuli that drive the modifications that lead to cartilage and bone damage.
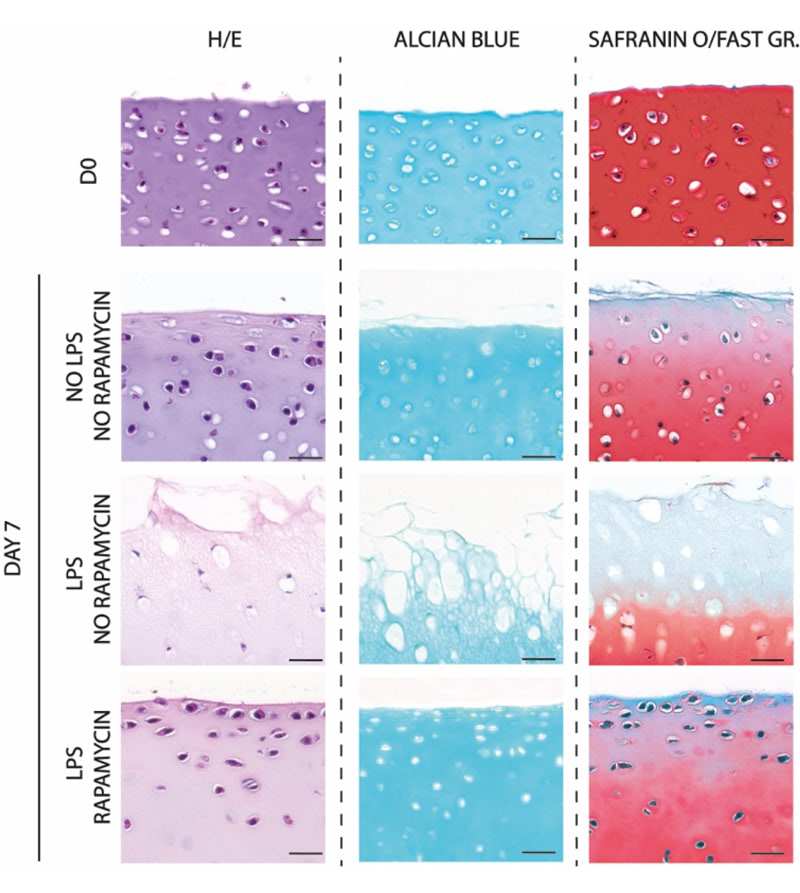
Recent findings highlighted the relevant influence of gut-resident microbiome in the regulation of inflammatory process in the body. In the particular case of the OA, the scientific community has given importance to the direct axis connecting gut and joint. Several studies found a correlation between pro-inflammatory microbiome-derived metabolites and osteoarthritis in humans. Interestingly, lipopolysaccharide (LPS) is among the most active bacterial metabolite in evoke an immune-mediated inflammatory response in joints.
In this context, our work is intended to deeply explore the LPS induced damage at tissue and cellular level and its role in osteoarthritis.
Impact:
Several in vitro studies have been conducted on chondrocytes cells culture to clarify the correlation between LPS and osteoarthritis. However, this approaches lack in relevant information regarding the effect of LPS at macroscopic scale.
With this work we aim to develop an OA model inducing inflammation on healthy native osteochondral explants to observe the effect of a pro-inflammatory stimuli at cellular and tissue level. Remarkably, our model realistically reproduces at the micro and macro scale the physiological joint environment, configuring as an advanced drug screening platform. Such technology opens the way to improve the drug screening process, promoting the discovery of new potential pharmacological therapies toward OA.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic area:
Products:
Drugs – Biomarkers – medical devices & tissue engineering
Collaborations:
- Partner of the European project Oactive – H2020: Lead partner: University of Nicosia;
- other partners on www.oactive.eu
- Bioengineering and Biomaterials Laboratory, Children’s Hospital of Philadelphia (CHOP), Philadelphia, Stati Uniti
- Dept. of Pediatrics, Perelman School of Medicine, University of Pennsylvania (UPenn), Philadelphia, Stati Uniti
- Dept. of Bioengineering, School of Engineering and Applied Sciences, University of Pennsylvania (UPenn), Philadelphia, Stati Uniti
- Center for Cellular and Molecular Engineering, Dept. of Orthopaedic Surgery, University of Pittsburgh, Philadelphia, Stati Uniti
Scarica il pdf del progetto
