iRhom2: a new therapeutic target in osteoarthritis?
Abstract
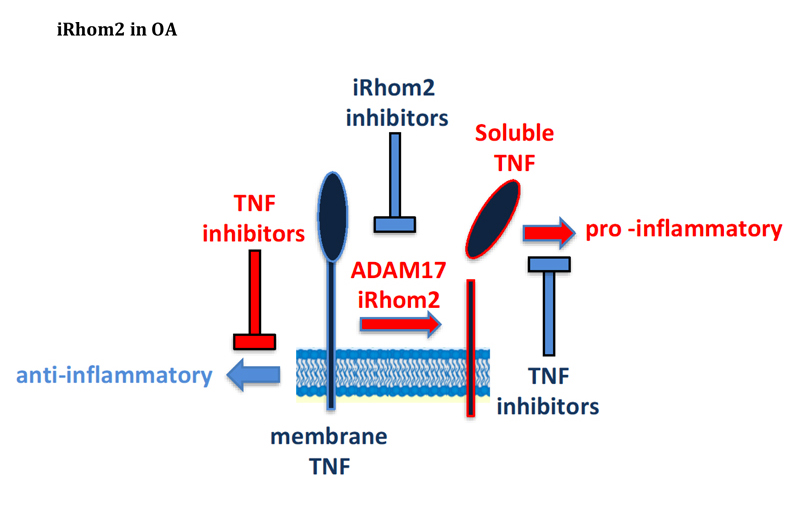
Osteoarthitis (OA) is a debilitating disease causing pain and stiffness. OA is characterized by breakdown of articular joint, due to the aberrant activity of MMPs and ADAMTSs. The endocytic receptor LRP1 controls turnover of these proteinases, thus its inactivation by ectodomain shedding contributes to development of the disease. In addition, the proinflammatory cytokine TNF plays a role in its progression by enhancing the expression of metalloproteinases. Similar to LRP-1, TNF is proteolytically released by ADAM17, and this cleavage elicits its pro-inflammatory potential. It is clear how inhibition of ADAM17 may lead to beneficial effects in OA progression by preventing LRP-1 and TNF shedding, thus enhancing metalloproteinase turnover and diminishing their expression, respectively. Nevertheless, ADAM17 cleaves more than 80 different proteins, and, as a consequence, its complete inhibition leads to their dysregulation with detrimental side-effects. iRhom1 and iRhom2 are essential regulators of ADAM17, in that they guide the enzyme maturation through the secretory pathway and direct its proteolytic activity towards specific substrates. By using unbiased secretome analysis, we found that ADAM17-mediated shedding of TNF and LRP-1 is specifically mediated by iRhom2, with iRhom1 that is not able to compensate. Thus, pharmacological inhibition of iRhom2 can be protective in OA, with lower risk of side effects.
Impact:
The proposed project will investigate the role of iRhom2 in the context of OA. iRhom2 is an ER trafficking protein that guides the maturation of ADAM17, a protease with a crucial role in development and inflammation. Although based on solid proteomic data, the proposed project is highly innovative. The major expected result will be the amelioration of OA progression in the absence of iRhom2. In addition, this study plans to generate a molecule that is able to block function of iRhom2, and therefore TNF release. It is expected, that upon a positive outcome of the primary objectives, multiple applications will arise. Indeed, implication of TNF and iRhom2 on the pathogenesis of inflammatory and neurodegenerative diseases, such as rheumatoid arthritis and Alzheimer’s, is already proven, and our inhibitory molecule can find an application in the therapy of these diseases. This will significantly promote multidisciplinarity among different medical specialties and research topics. It has recently emerged that iRhom2 and its homologue iRhom1 can direct ADAM17 activity towards specific substrates, but this area of investigation is still on its infancy. Our study will lead to a comprehensive analysis of those proteins that are processed by ADAM17 in an iRhom1 or iRhom2-dependent manner. Thus, it will provide further insight into the iRhom biology, revealing new functional and structural properties of these proteins and the mechanism by which they regulate ADAM17 substrate-selectivity.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic Areas:
Product:
Drugs and Biologics
Collaborations:
- Institute of Aging and Chronic Diseases, University of Liverpool, Liverpool, Regno Unito
- Pharmacy Department, Università di Pisa, Pisa, Italia
- German Center for Neurodegenerative Diseases (DZNE), Monaco, Germania
Scarica il pdf del progetto
