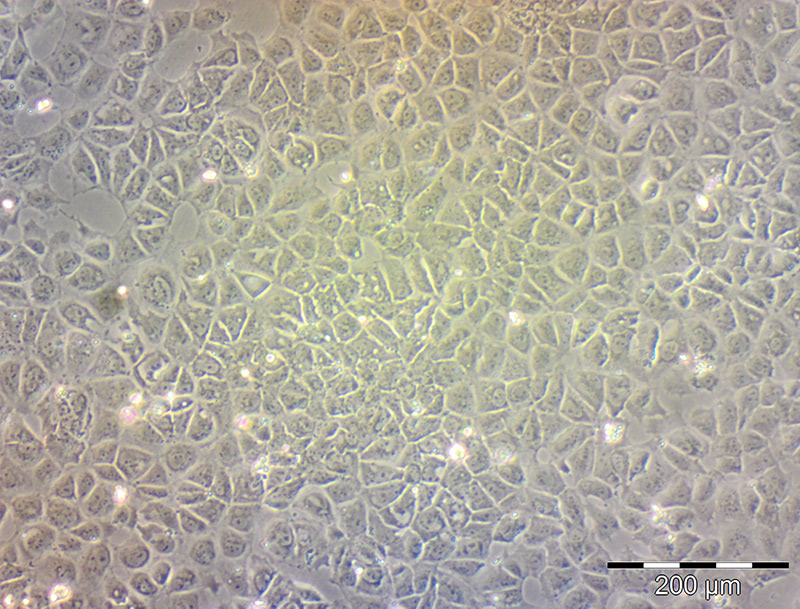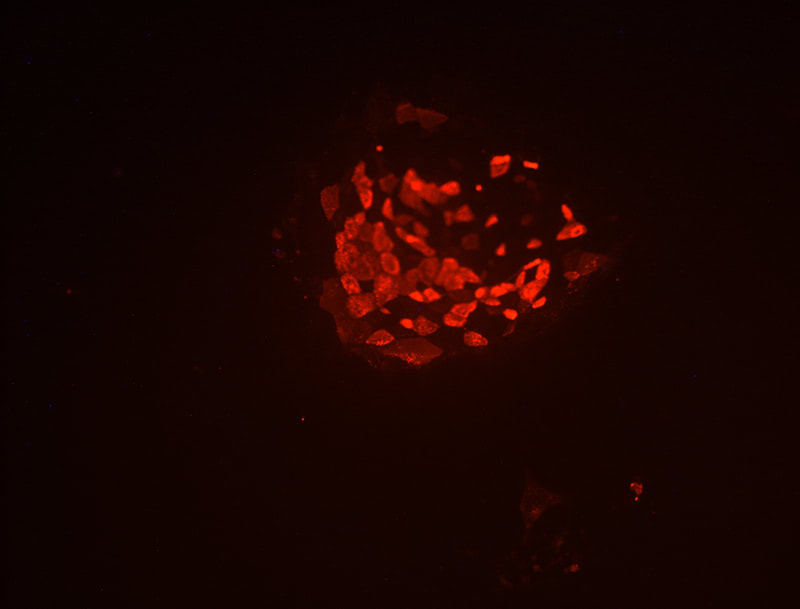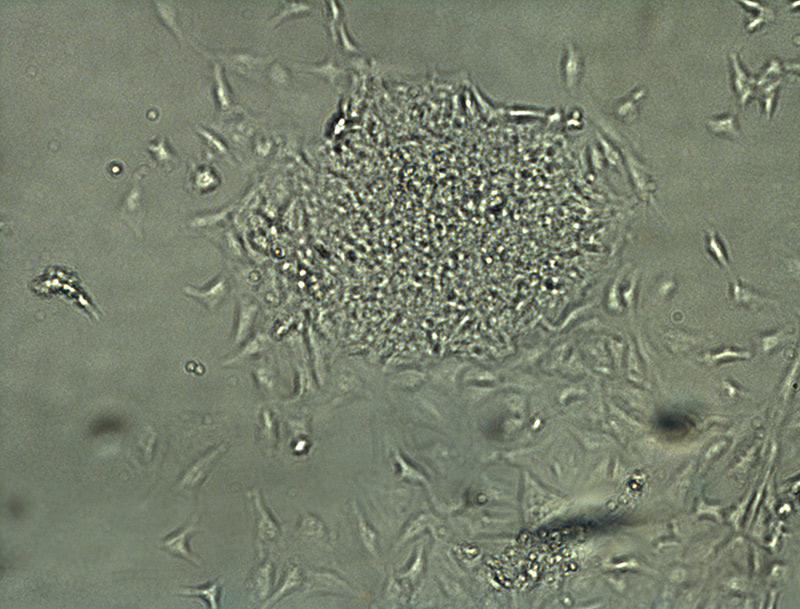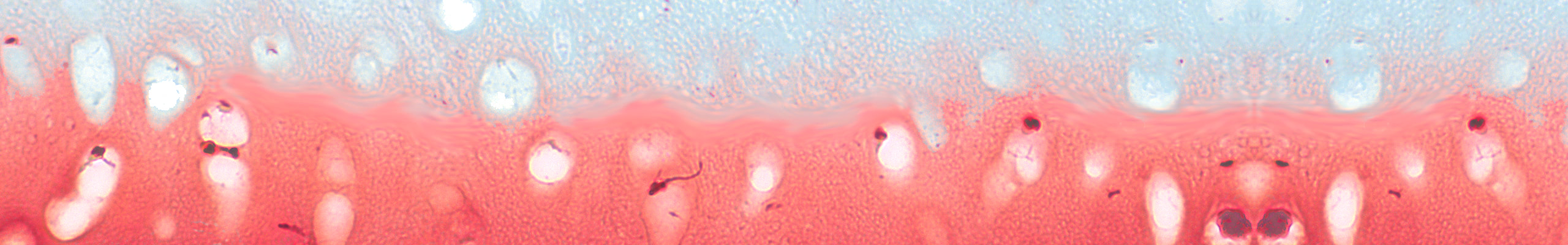
Development of a reliable ex vivo osteoarthritis model responsive to mTORC1-inhibitors via oxidative stress reduction and autophagy promotion
Abstract
Osteoarthritis (OA) is a chronic joint disease, which causes pain, swelling, deformation, and in the most severe cases, hypomobility of the joint. Intuitively, these pathologic conditions seriously get in the way for daily movements, and cause invalidating conditions due to the need for supporting assistance1. Hyaline cartilage is the more heavily involved joint area. However, the underlying mechanisms responsible of osteoarthritis are complex and interconnected, and interest all joint structures such as subchondral bone, synovium, muscles, and ligaments2,3. Recent epidemiologic analyses4 pointed out the age-related nature of osteoarthritis, and highlighted that the OA incidence in 75 years old population is 4 times higher than in 40 years old people for hip osteoarthritis, and 14 times higher for knee osteoarthritis. Moreover, age-related comorbidities with an incidence between 14% and 20% has been reported5. The point of contact was found in the intestinal disorder known as leaky gut (LG), which is one of the most frequent OA comorbidities age-related6. The etiology of leaky gut is reportedly connected with a dysregulation of the homeostasis in the intestinal epithelium, which is mainly composed of enterocytes covered by a layer of protective mucus. Here, cells are interconnected each others through tight junctions, a cell-cell connection which reduce to zero intercellular gaps blocking paracellular translocation of substances (Figure 1). LG is caused by the partial or total laxity of tight junctions, which promote the diffusion in the bloodstream of LPS produced by resident microbiota triggering systemic inflammatory responses (SIR) that damage joint cartilages
Impact:
In the last decade, interdisciplinary studies involving regenerative medicine, molecular biology, and pharmaceutical chemistry, are directed to discover new pathways involved in OA to be used as target for innovative pharmacological approaches. However, the challenge is particularly hard due to heterogenous OA-related molecular mechanisms involving a wide plethora of biochemical factors. Very recently, the pathways activated by the interaction of lipopolysaccharide (LPS) with its extracellular receptor (toll like receptor 4, TLR4) in chondrocytes has been strictly correlated with the onset of osteoarthritis. Our work is included in this complex scenario, and aims to create a stimuli responsive 3D ex vivo model mimicking the leaky gut-related OA. Our encouraging results suggest that our model will have a strong impact in the development of new pharmacological therapy targeting osteoarthritis secondary to TLR-4 receptor activation. The model will be used as a reliable and realistic bench test for new small molecules as well as to test innovative drug delivery systems (DDS).
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic Areas:
Product:
Medical Devices & Tissue Engineering
Collaborations:
- Bioengineering and Biomaterials Laboratory, Children’s Hospital of Philadelphia (CHOP), Philadelphia, USA
- Dept. of Pediatrics, Perelman School of Medicine, University of Pennsylvania (UPenn), Philadelphia, USA
- Dept. of Bioengineering, School of Engineering and Applied Sciences, University of Pennsylvania (UPenn), Philadelphia, USA
- Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione (IRCCS ISMETT), Palermo, Italia
- Reparto di chirurgia ortopedica, clinica Buccheri La Ferla, Palermo (Italia)
Scarica il pdf del progetto