The human umbilical cord. From discarded tissue to a possible source of care
Abstract
The PROMETEO Project aims to evaluate the use of perinatal tissue-derived cells for the treatment of chronic inflammatory pathologies causing organ failure. Among these, in addition to liver disease (a major causes of public health problems and death worldwide), there are also dysfunctions of the uterine wall that negatively affect the success of a pregnancy due to a thinning of the endometrial component, poorly receptive.
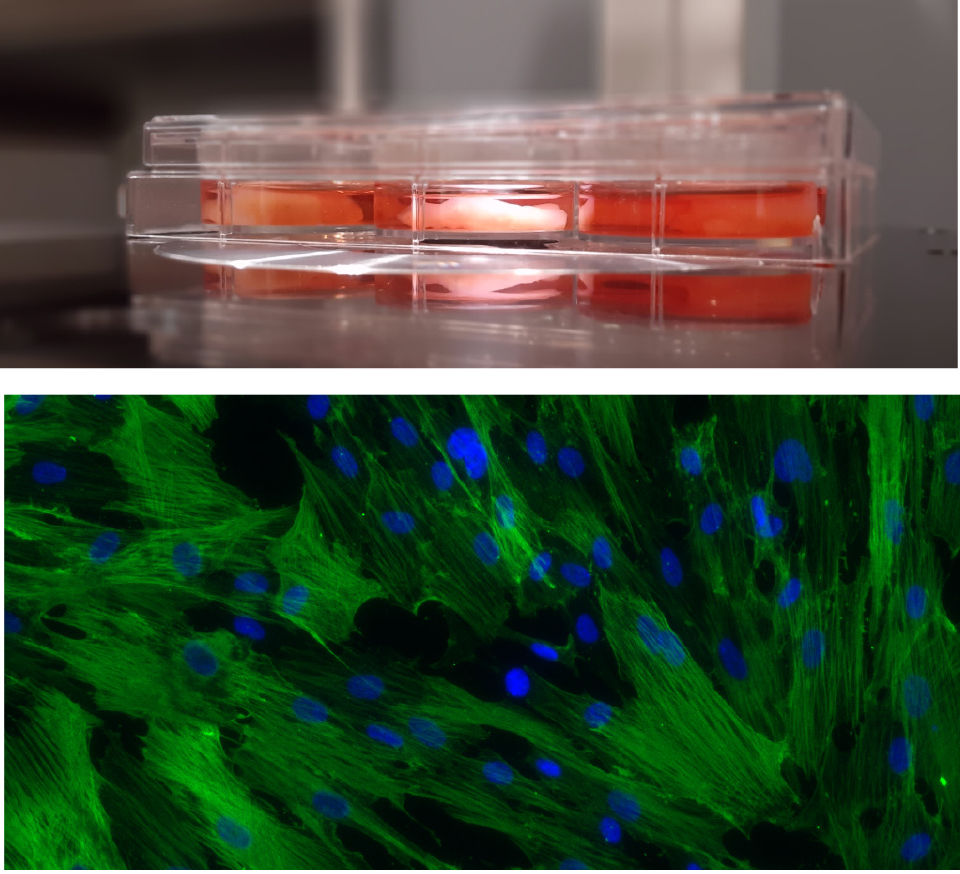
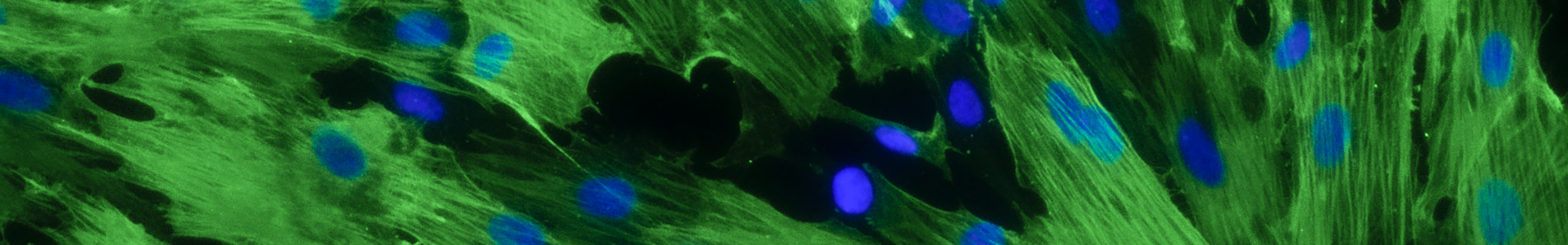
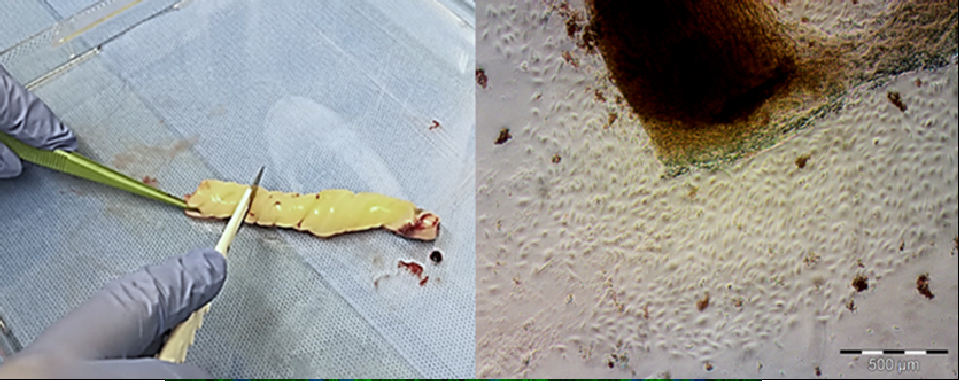
Umbilical cord mesenchymal stromal cells (hUC-MSCs) are extensively used in recent studies on the treatment of inflammatory states (for example, the consequences of Covid-19). The high number of stromal cells in a tissue considered as a waste, the positive impact related to ethical problems, and their immunoregulatory, anti-inflammatory and, presumably, stimulating cell regeneration, even by releasing of extracellular vesicles (EVs), make the cord a promising source of cellular products for advanced therapies.
The analyses on EVs and microRNAs (miRNAs) contained therein, in addition to the soluble factors released by hUC-MSCs in the extracellular environment, and the development of experimental models that reflect the clinical pathological aspect of the endometrium, will allow establishing a possible reparative phenomena and functional recovery of the damaged tissue.
Impact:
If the experiments on in vitro models show that hUC-MSCs and their derivatives (EVs and/or soluble factors) are capable of promoting tissue regeneration and modulating inflammatory processes affecting the endometrial tissue, hUC-MSCs could be used in clinical settings for curing patients otherwise subjected to treatments that have not yet given full efficacy (endometrial scratch, immunoglobulins, glucocorticoids, anticoagulants). The high number of MSCs obtainable from each cord, the ability to activate the reparative, anti-fibrotic, anti-bacterial, and pro-angiogenic mechanisms generated by the cells or, possibly, by their secretion products (immunosuppressive molecules and miRNAs contained in EVs), could be at the basis of a high-number cell production for repairing therapy programs and restoration of tissue function. Developing cellular products, according to GMP standards, would lead to the generation of a medicinal product alternative to the usual pharmacological therapies that, in the end, can still induce serious cell damage. This would therefore favor not only a clear improvement in the quality of patients’ life but also a reduction of therapeutic failures, the number of embryonic implants, and health and hospital management costs.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic Areas:
Product:
ATMP (Advanced Therapy Medicinal Products)
Collaborations:
- Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione (IRCCS ISMETT), Palermo, Italia
- Casa di Cura Candela, Palermo, Italia
Scarica il pdf del progetto