The role of repetitive sequences in cellular senescence and aging
Abstract
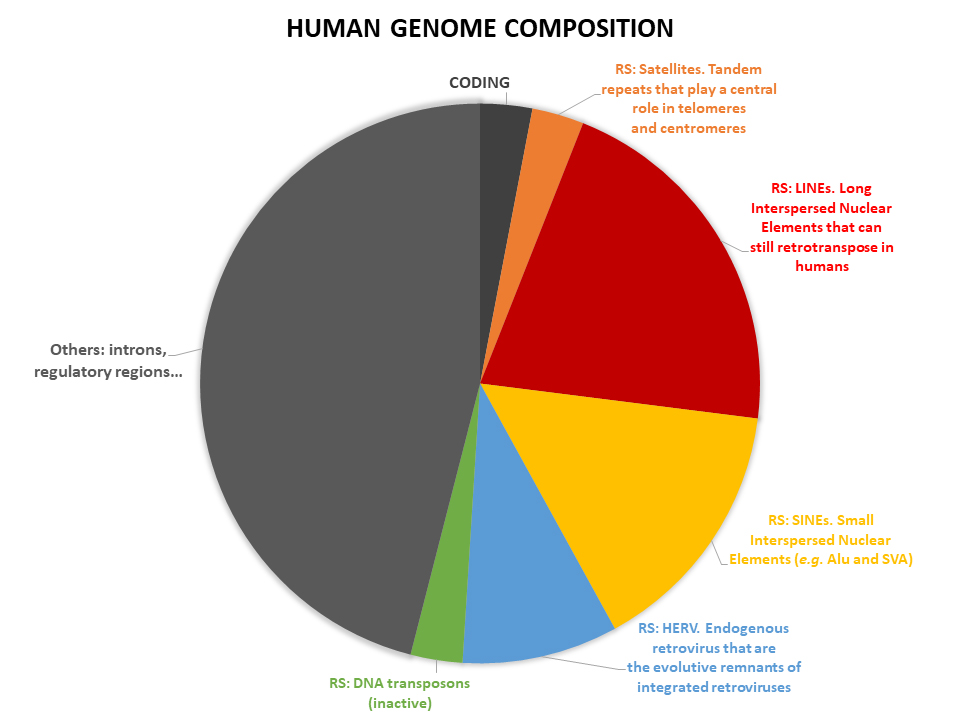
Repetitive DNA sequences (RS) represent about half of the human genome. Despite RS have been considered for long just “junk DNA”, now it is clear that RS have a role in almost every aspect of human biology, especially in aging and aging related diseases, but also in cancer and development. The role of the RS has been often overlooked because RS are intrinsically difficult to study and dedicated experimental procedures and bioinformatics pipelines are needed; procedures and pipelines we are able to perform. Among many other roles in cell biology, RS seem to play a specific central role in triggering cellular senescence that in turn is one of the main factors driving the human aging process and progress. Cellular senescence is a state of permanent cell cycle arrest that induce a proinflammatory microenviroment in their surroundings, that is usually reset by the removal of senescent cells due to macrophage activity. During aging instead senescence cells accumulate, and in turn induce a chronic mild inflammatory state that is probably at the very root of many diseases of the elders, such as cardiovascular and degenerative diseases, but also tissue remodeling as fibrosis or even cancer. A better understanding of the crosstalk between RS and cellular senescence can shed light to novel biomarkers, therapeutic targets and clinical approaches for the treatment of aging and aging related diseases, and over.
Impact:
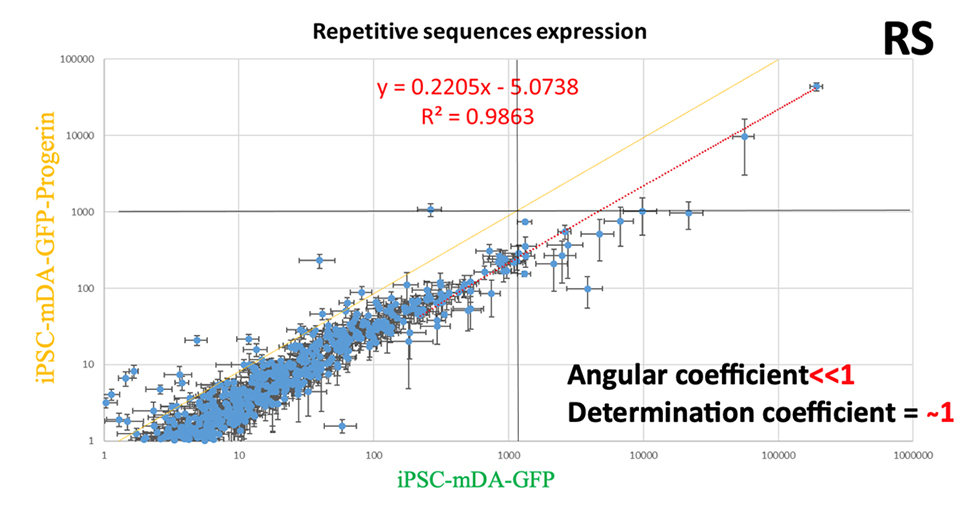
A plethora of evidences are accumulating in suggesting that RS play a central role in aging and aging related diseases. In particular, RS at the telomeres are strictly interlaced with the replicative senescence and at centromeric level with chromosomal stability. It is known that RS are under a strict epigenetic control mechanism based on CpG DNA methylation. During aging, this epigenetic landscape is subverted, and the sequence homologies of RS became a hub for homology directed recombination thus causing in turn genomic instability, DNA damage, senescence and even cancer transformation. Moreover, RS usually carry regulatory sequences, thus their deregulation, and/or mobilization, can alter the transcriptional profile of the cell. RS activation has been suggested as a general feature of aging cells that acquire the senescent phenotype and recently it has been proved that RS repression in senescent cells triggers the inflammation associated with aging (inflammaging) via the cytosolic DNA sensing pathway (cGAS-STING), a component of the innate immune system against virus infection. By all these reasons, RS are relevant potential targets for the treatment of age-associated disorders.
Pipeline
-
CLINICAL
NEED -
DISEASES
ANALYSIS - DISCOVERY
-
PRECLINICAL
VALIDATION -
PRECLINICAL
DEVELOPMENT -
CLINICAL
STUDIES
Principal Investigator
Contact
Therapeutic Areas:
Product:
Biomarcatori, farmaci biologici
Collaborations:
- Istituto per la Ricerca e l’Innovazione Biomedica (IRIB) – CNR, Palermo, Italia
- Dipartimento di Scienze e Tecnologie Biologiche Chimiche e Farmaceutiche (STEBICEF) – UNIPA, Palermo, Italia
- Dipartimento di Scienze Economiche, Aziendali e Statistiche (SEAS) – UNIPA, Palermo, Italia
- Dipartimento di Biologia Computazionale (CSB) – University of Pittsburgh, Pittsburgh, Stati Uniti
Scarica il pdf del progetto